Solidphase peptide synthesis (SPPS) remains the most efficient approach for the chemical preparation of standard peptides. Our peptide chemists team has many years experience in peptide synthesis of various length,
► Standard sequence length: 5 to 30 aa.
► Routine sequence length from 31 to 60 aa.
► Challenging sequence length from 61 to 100 aa.
Because peptides of length over 40 aa have raised important research interests, our customers’ request for long peptides (40 aa up to 80-100 amino acids) is increasing. While being challenging, such large molecules could be successfully synthesized at Genosphere Biotechnologies by stepwise solidphase peptide synthesis.
For example, dipeptide repeats have been implicated in a number of disorders. We have successfully prepared many long to very long direpeats, including proline-containing poly(Pro-lys)20 that have been associated with amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD).
The expansion of GGGGCC repeats within the first intron of C9ORF72 constitutes the most common cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Through repeat-associated non-ATG translation, these expansions are translated into dipeptide repeats (DPRs), some of which accumulate at nucleoli and lead to cell death.
Importantly, the two dipeptide repeats (DPRs) that contain arginine, poly(GR) and poly(PR), are toxic to cells even when added to culture media as well as in model organisms, providing a direct model to explain the pathogenicity of C9ORF72 mutations. Arginine-containing DPRs enter into cells, where they concentrate at nucleoli altering their size and several nucleolus-related functions such as rRNA biogenesis or mRNA splicing, ultimately leading to cell death.
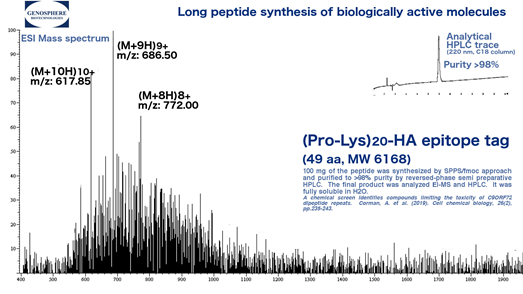
Figure 1. Synthesis and purification of (PR)20 peptide with C-terminal HA epitope tag:
PRPRPRPRPRPRPRPRPRPRPRPRPRPRPRPRPRPRPRPRYPYDVPDYA
Cell chemical biology, 26(2), pp.235-243.
However, there is a size limit to the SPPS capabilities. Indeed, with increasing peptide length the stepwise and linear nature of SPPS leads to lower synthesis cycle yields. It is principally the consequence of lower accessibility to the reaction site from peptide chain aggregation and steric hinderance which leads to truncated sequences and unwanted side products. The accumulation of these byproducts puts a strain on the purification steps to isolate the desired full length peptide. Depending on the actual sequence of the peptide, the crude synthesis may be very impure and cannot be purified by standard chromatographic methods.
The size limitation has prompted the development of new chemical ligation methods to assemble shorter peptides into larger polypeptides and even proteins. Early work in this area relied on the condensation of side chain protected peptides. This approach has been successful to some extent for the synthesis of therapeutic peptides. However, the poor solubility of crude protected fragments and the occurrence of epimerization of the Cterminal amino acid at the activation step has rendered this strategy largely impractical.
A key solution to these issues was provided through the development of peptide ligation chemistries that facilitate the formation of native peptide bonds between completely unprotected peptide fragments: the Native Chemical Ligation (NCL). NCL has played a key role in enabling the total synthesis and semi-synthesis of increasingly complex peptide and protein sequences. Classical NCL proceeds through the chemoselective reaction of two unprotected polypeptide chains in aqueous solution at close to neutral pH and is made possible by the presence of a thioester moiety on the C-terminus of the N-terminal peptide fragment and a cysteine residue on the N-terminus of the C-terminal peptide fragment. The reaction yields an amide bond adjacent to cysteine at the ligation site (Figure 2).

Figure 2. Classical Native Chemical Ligation reaction scheme
Importantly, Genosphere Biotechnologies can prepare the unprotected peptide fragments by optimized solid phase peptide synthesis in high purity and high yield and further characterize them by reversed-phase high-performance liquid chromatography (RP-HPLC) and electrospray mass spectrometry (ESI-MS). Genosphere Biotechnologies has a thorough experience in preparing the C-ter thioester NH2-[peptide]-CO-SR fragments. Figure 2 shows the ESI-MS of the C-thioester prepared at purity of >95% in high yields.

Figure 3. Synthesis of a C-thioester modified 21mer for use in a Native Chemical Ligration reaction.
Other advances in the field of chemoselective peptide bond formation include α-ketoacid-hydroxylamine ligation, and serine/threonine ligation.
These approaches have enabled the chemical synthesis of numerous peptides and proteins that were previously out of reach to the classical recombinant methods or SPPS. They have thus played a key role in addressing a number of important questions in biology, pharmacology, and medicine. However, at this point, NCL remains a costly strategy, and cannot be applied as a routine method for synthesizing long peptides for research purposes.
For More Information about Long Peptide Synthesis Visit https://bit.ly/3AbvM5T
References
- Synthesis of proteins by native chemical ligation.
Dawson PE et al. (1994) Science 266(5186):776-9.
- Native chemical ligation of polypeptides.
Camarero JA & Muir TW. (2001) Curr Protoc Protein Sci. Chapter 18:Unit18.4
- Advances in Fmoc solid-phase peptide synthesis.
Behrendt R et al. (2016) J Pept Sci. 22(1):4-27.
- Recent advances in the preparation of Fmoc-SPPS-based peptide thioester and its surrogates for NCL-type reactions.
Li, H. & Dong, S. (2017) Science China Chemistry, 60(2), 201-213.
- Native Chemical Ligation of Peptides and Proteins
Cistrone PA et al. (2019) Curr Protoc Chem Biol. 11(1): e61.
- Native Chemical Ligation via N-Acylurea Thioester Surrogates Obtained by Fmoc Solid-Phase Peptide Synthesis.
Palà-Pujadas J & Blanco-Canosa JB. (2020) Methods Mol Biol. 2133:141-161.

