Solidphase peptide synthesis (SPPS) remains the most efficient approach for the chemical preparation of standard peptides. Our peptide chemists team has many years experience in peptide synthesis of various length,
► Standard sequence length: 5 to 30 aa.
► Routine sequence length from 31 to 60 aa.
► Challenging sequence length from 61 to 100 aa.
Because peptides of length over 40 aa have raised important research interests, our customers’ request for long peptides (40 aa up to 80-100 amino acids) is increasing. While being challenging, such large molecules could be successfully synthesized at Genosphere Biotechnologies by stepwise solidphase peptide synthesis.
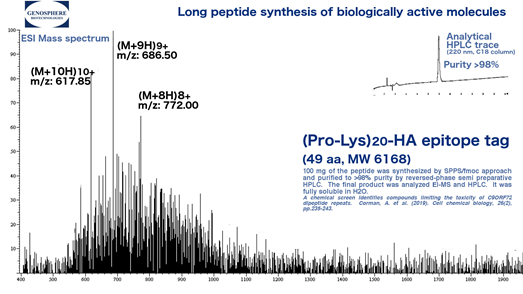
For example, dipeptide repeats have been implicated in a number of disorders. We have successfully prepared many long to very long direpeats, including proline-containing poly(Pro-lys)20 that have been associated with amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD).
The expansion of GGGGCC repeats within the first intron of C9ORF72 constitutes the most common cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Through repeat-associated non-ATG translation, these expansions are translated into dipeptide repeats (DPRs), some of which accumulate at nucleoli and lead to cell death.
Importantly, the two dipeptide repeats (DPRs) that contain arginine, poly(GR) and poly(PR), are toxic to cells even when added to culture media as well as in model organisms, providing a direct model to explain the pathogenicity of C9ORF72 mutations. Arginine-containing DPRs enter into cells, where they concentrate at nucleoli altering their size and several nucleolus-related functions such as rRNA biogenesis or mRNA splicing, ultimately leading to cell death.
Figure 1. Synthesis and purification of (PR)20 peptide with C-terminal HA epitope tag:
PRPRPRPRPRPRPRPRPRPRPRPRPRPRPRPRPRPRPRPRYPYDVPDYA
Cell chemical biology, 26(2), pp.235-243.
However, there is a size limit to the SPPS capabilities. Indeed, with increasing peptide length the stepwise and linear nature of SPPS leads to lower synthesis cycle yields. It is principally the consequence of lower accessibility to the reaction site from peptide chain aggregation and steric hinderance which leads to truncated sequences and unwanted side products. The accumulation of these byproducts puts a strain on the purification steps to isolate the desired full length peptide. Depending on the actual sequence of the peptide, the crude synthesis may be very impure and cannot be purified by standard chromatographic methods.
Cyclic peptides have attracted a lot of attention in recent years, especially in the area of drug discovery, as many naturally occurring cyclic peptides with a variety of biological activities are being discovered. Chemical synthesis of cyclic peptides is essential when studying their structure–activity relationships. Conventional cyclic peptide synthetic methods via direct coupling have been refined. Genosphere Biotechnologies offers a versatile platform for synthesis of cyclic peptides and constrained peptides including disulfide bridged peptides, Nter to Cter lactam cyclisations, hydrocarbon stapling, and specialised modifications such as internal lactam or lacton cyclizations.
The advance in cyclic peptide synthesis will benefit the biological and pharmacological study this important new class of macrocycles with potentials in numerous fields, notably in therapeutics.