We measure gas pressure in everyday life when we use a barometer to check the outside atmospheric pressure or a tyre gauge to check the pressure in a bike tube. If you break it, it is about measuring the macroscopic physical attribute of a big number of gas molecules that aren’t visible to the human eye. The pressure we’re measuring is the result of individual gas molecules hitting other objects, such as the container’s walls, at the molecular level.
The working principle of a zirconium dioxide O2 sensor is to measure the partial pressure of oxygen in a mixture of gases. Most oxygen sensors such as the portable oxygen analyzer or the other medical gas analyzer in the market measure oxygen concentration to monitor the proper functioning of the equipment. Handheld oxygen analyzer is commonly used across the medical sector as well as for some other commercial uses as well. So, knowing about the partial pressure can be actually helpful.
So, what exactly is partial pressure? When it comes to the O2 sensor’s functioning principle, we are frequently asked this topic. For individuals interested in oxygen concentration, this article will cover the definition of partial pressure, the physics behind it, how to compute partial pressure, and how to convert oxygen partial pressure into volumetric content.
The Ideal Gas Equation applies equally well to mixtures of gases as it does to pure gases because it is based purely on the quantity of particles and not on the identity of the gas. Boyle, Gay-Lussac, and Charles conducted their early tests with a gas mixture (a mix of ordinary air). To deal with gas mixtures, we merely need to learn about partial pressure, which was invented by the famous English chemist John Dalton (1766-1844). Dalton correctly reasoned that gases’ low density and high compressibility indicated that they were primarily made up of empty space; as a result, he inferred that when two or more different gases occupy the same volume, they operate completely independently of one another.
Dalton’s law of partial pressures
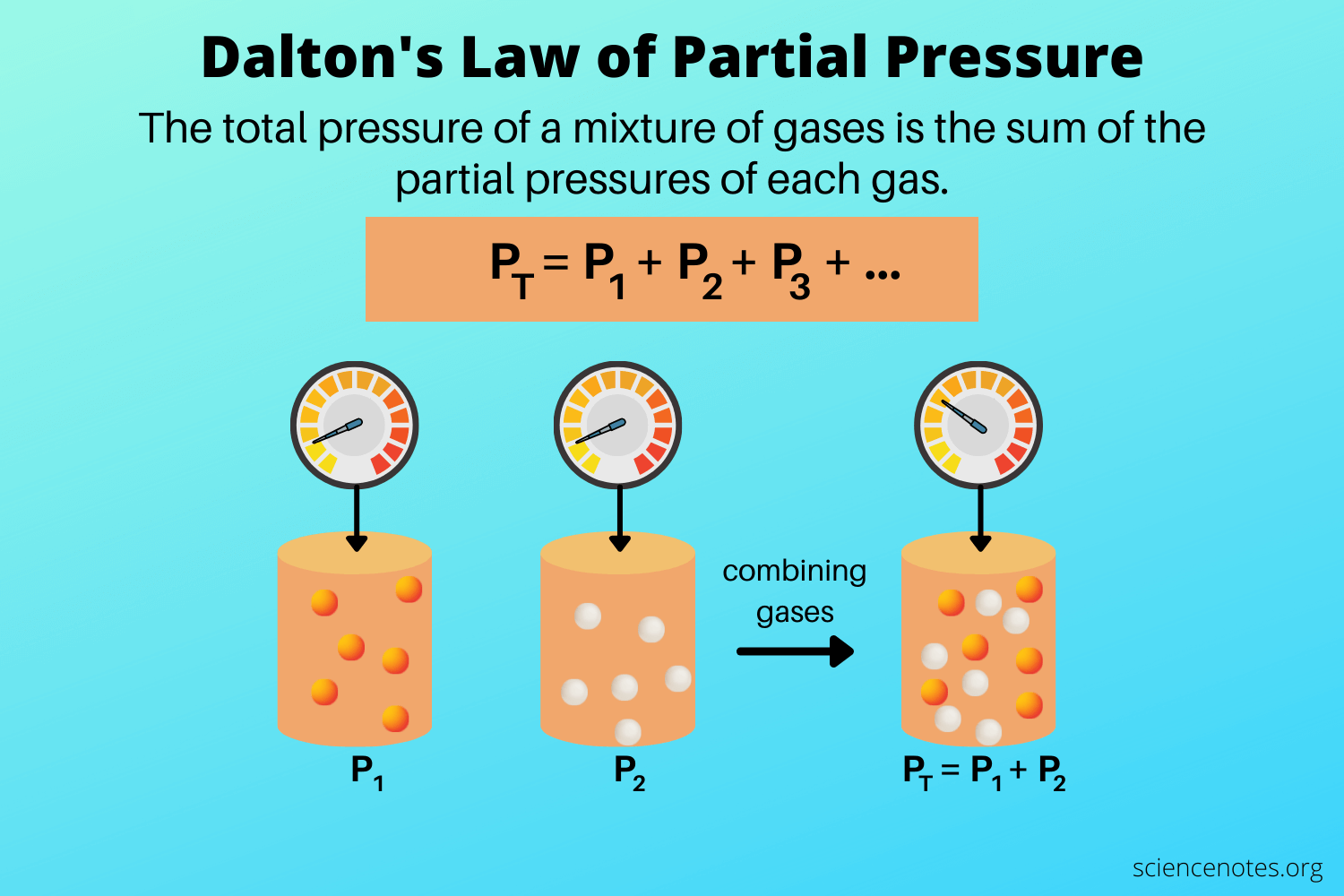
Dalton’s law states that the total pressure of a mixture of gases is the sum of the partial pressures of its components and is shown as
P Total = P gas 1 + P gas 2 + P gas 3…
And the partial pressure of each gas is the pressure that the gas would exert if it was the only gas in the container. It works on the assumption that there are no attractive forces between the gases.
The theory of the o2 sensor working principle is that the total pressure (Ptotal) of a mixture of ideal gases is equal to the sum of the partial pressures (Pi) of the individual gases in that mixture. It is shown by –
![]()


